Nature of matter
Matter is the basic structural component of the universe.It is anything that has mass and occupies space i.e they have mass and volume. Examples of matter includes plants and animals around us, the food we eat, the liquid we drink and even the air we breathe.
The mass of an object is the quantity of matter an object contain and is the same everywhere. The S.I unit of mass is Kilogram (Kg).
Properties of matter
Properties of matter are divided into two which are:
1) Physical properties: This are properties associated with physical changes. Examples are boiling point, melting point, density, hardness, malleability, crystalline form as well as properties which may be detected by our senses such as colour,odour and taste.
2)Chemical properties: These are properties that are associated with chemical changes(i.e chemical reactions) that matter undergoes such as rusting of iron.
Properties that are not characterized of any particular type of matter such as mass, length and temperature are known as extrinsic properties.
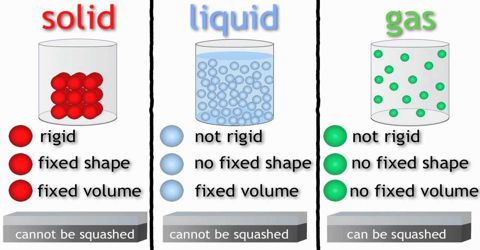
State of matter
All matter can change from one state to another when heated or cooled e.g when water is heated it changes from liquid to steam,when water is cooled it changes from water to Ice..
Types of change
1)Physical change: It is the change that is easily reversible and produces no new substance e.g dissolution of common salt In water,because no new substance is formed,the salt can be recovered by evaporating the solution to dryness.
2)Chemical change: Chemical change is the transformation in which a new substance is formed and not easily reversible e.g burning of wood.
Differences between physical and chemical changes
1)Physical change does not involve heat change while Chemical change does.
2)In physical change, no new substance is formed while in Chemical change,new substances are formed.
3) Physical changes are easily reversible while in Chemical change it is not easily reversible.

